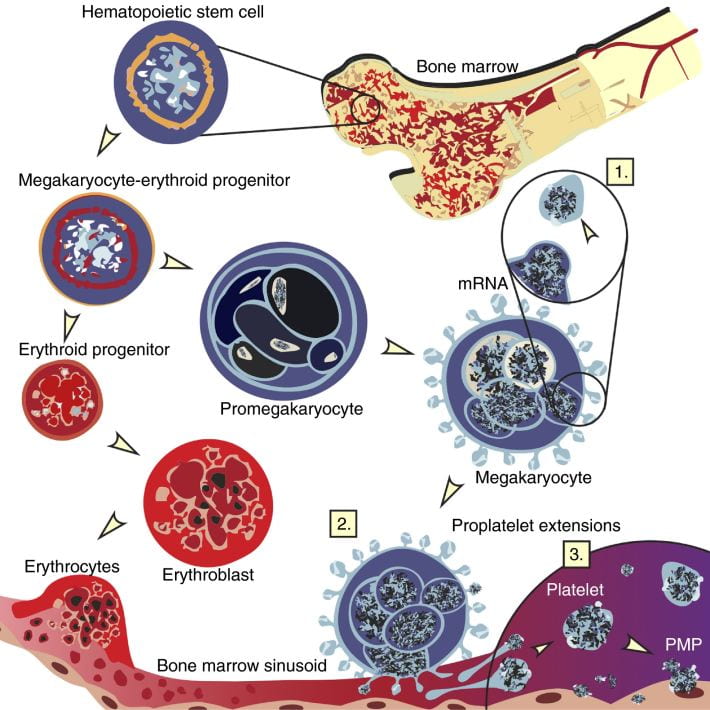
Inherited platelet disorders are usually classified by platelet parameters that often include morphology and size as well as mode of inheritance. This is particularly useful for the practicing hematologist when a diagnosis of a hereditary platelet disorder is being considered. Our understanding of the mechanisms of megakaryopoiesis and platelet biology have improved significantly over the last 2 decades and therefore these disorders may also be classified by the megakaryocyte pathway affected by the mutated gene responsible for the disease. This molecular classification is complementary to the clinical classification and may allow scientists to not only expand their knowledge about pathophysiology, but also to ask fundamental questions about similar pathways affected in other cells and potential targeted therapies.
Fisher et al. RPTH, 2018
With the completion of the Human Genome Project in 2003 and vast advances in sequencing technology in the last 2 decades, genome-wide research became available in a more cost-effective manner and several platelet disorders have been elucidated. Our lab, among others, have contributed to platelet genes discovery by reporting that mutations in NBEAL2 and ETV6 cause gray platelet syndrome and thrombocytopenia with predisposition to leukemia respectively. Over the last decade we have developed several genomic approaches to understand the molecular consequences of these genetic changes. Additionally, we employed cellular and animal models of these disorders in order to understand the biological significance of these genetic defects, and their potential impact on human disease.