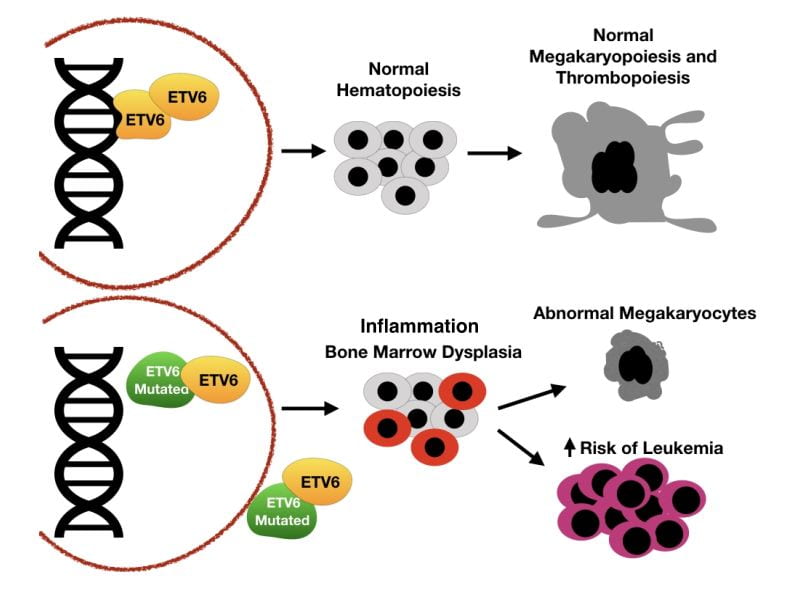
Recently, we found that mutations in ETV6 lead to mild thrombocytopenia with a bleeding diathesis, red cell macrocytosis, and predisposition to lymphoblastic leukemia.
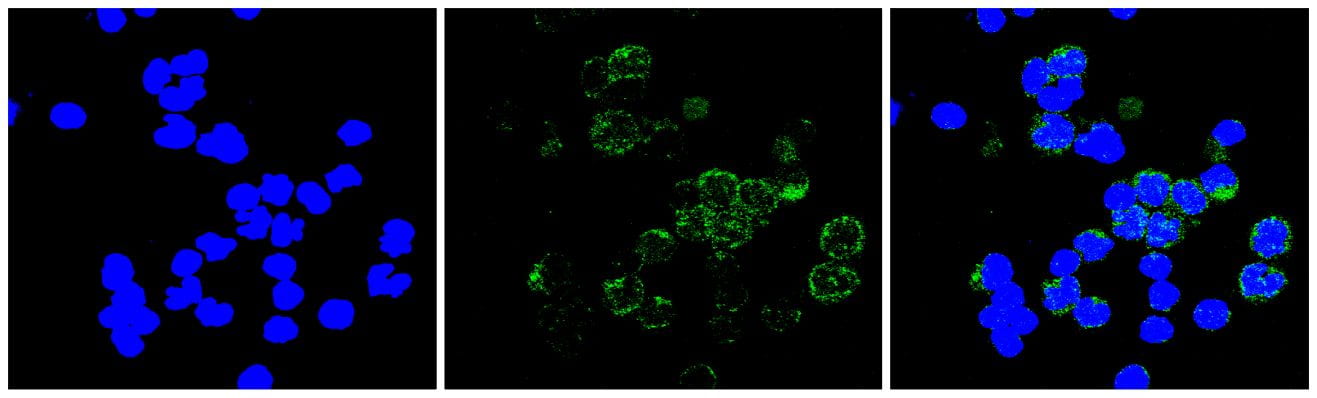
The mechanisms responsible for the thrombocytopenia and propensity for bleeding in patients with ETV6 mutations are unknown. We described families with missense mutations in the central domain (p.Pro214Leu) and the ETS DNA binding domain (p.Arg418Gly) of ETV6 that result in aberrant cellular localization of ETV6, decreased transcriptional repression, and impaired MK maturation. Deep sequencing of the platelet transcriptome revealed significant differences in mRNA expression levels between patients with the ETV6 p.Pro214Leu mutation and non-affected family members, indicating that ETV6 is critically involved in defining the molecular phenotype and function of platelets. Additionally, single cell RNA-sequence of peripheral mononuclear blood cells from these patients demonstrated significant changes in the expression patterns of mRNAs of Interferon (IFN) Response Genes, suggesting a critical role for ETV6 in maintaining bone marrow homeostasis.
With a combination of cellular and animal models we are investigating:
- The molecular mechanisms by which ETV6 regulates MK differentiation, platelet formation and function
- The contributions of ETV6 in modulating transcriptional events in MKs
- The consequences of ETV6 disruption in bone marrow homeostasis
This work is done in collaboration with Eric Pietras from University of Colorado, Jesse Rowley from University of Utah, and Christopher Porter from Emory University.

Marlie Fisher, MS
MD/PhD Student, University of Colorado Denver
Marlie Fisher is an MD/PhD student in the Di Paola lab studying germline mutations in ETV6 that drive aberrant megakaryocyte development and thrombocytopenia. She is a golfer and skier when she is not in the lab.
Further Reading
Fisher MH, Di Paola J. Genomics and transcriptomics of megakaryocytes and platelets: Implications for health and disease. Research and Practice in Thrombosis and Haemostis, July 2018; 2(4):630-639.
Di Paola J, Fisher MH. ETV6-related thrombocytopenia and platelet dysfunction. Platelets, May 2020.